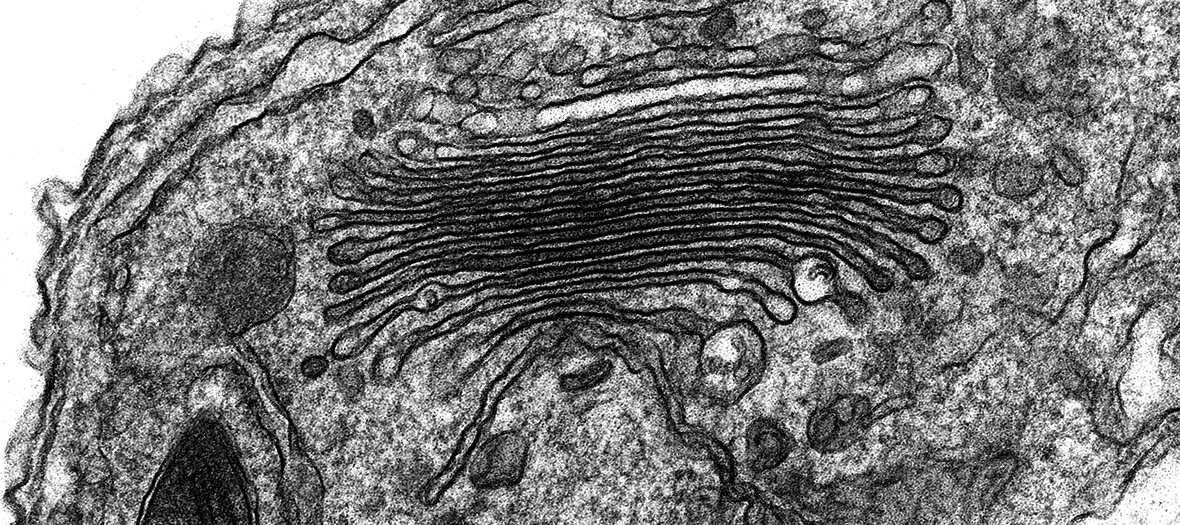
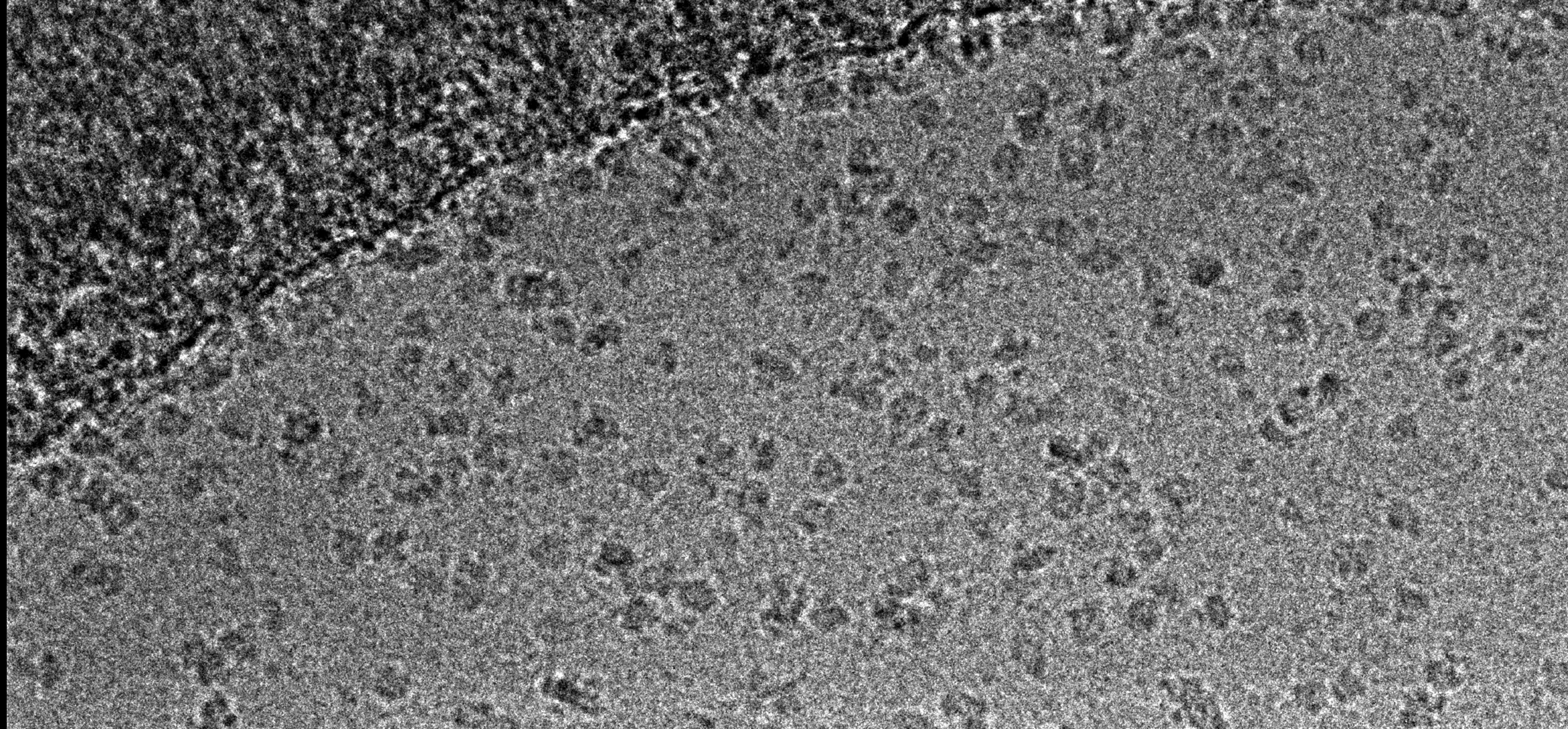
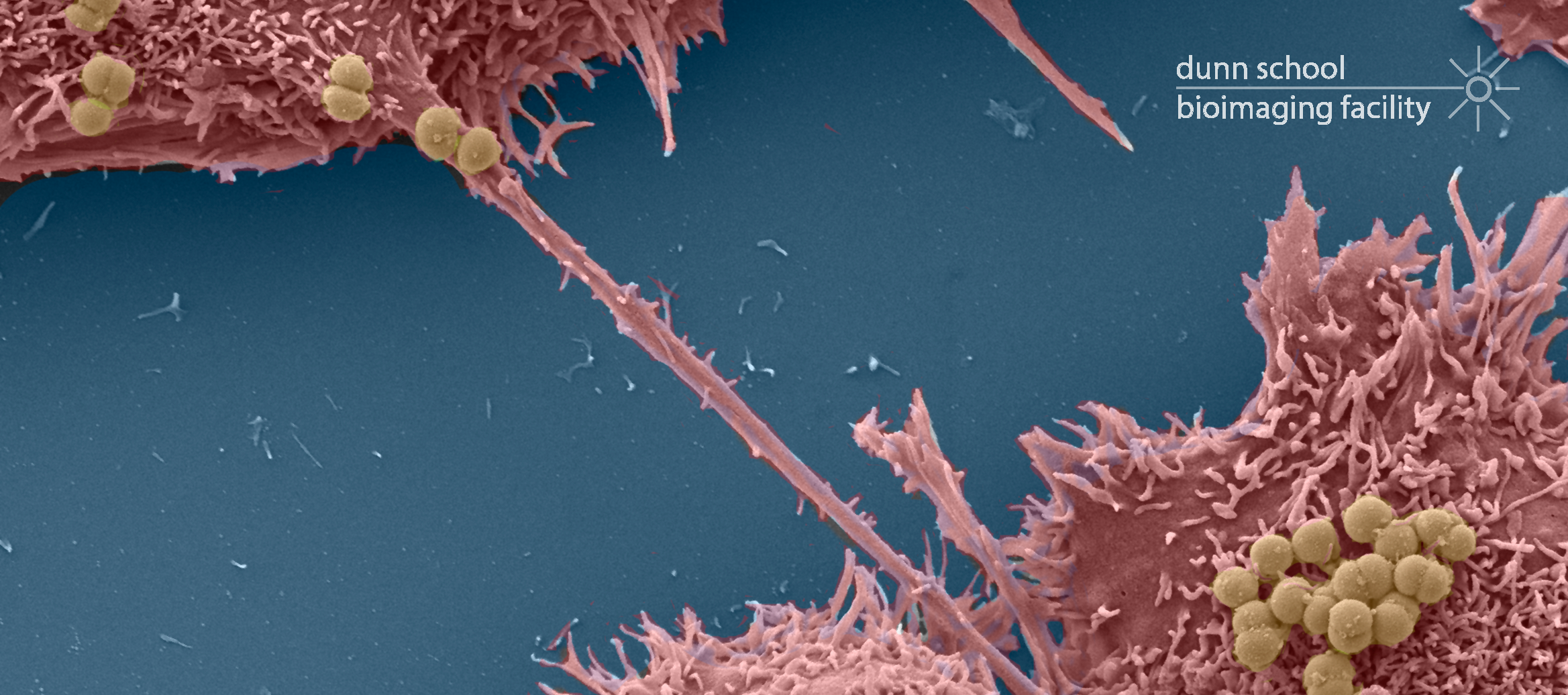
EM Specimen Preparation Laboratory
Room 214.00.21
The EM Specimen Preparation lab is equipped with the latest model instrumentation to facilitate processing of biological samples for EM using a range of different techniques, with dedicated expert staff on hand to train and advise users.
Reagents for TEM and SEM preparation:
- Stock solutions – 25% glutaraldehyde, 16% formaldehyde, 0.2M Sodium cacodylate buffer, 0.2M PIPES buffer, PBS, 2% and 4% Osmium tetroxide, 2% uranyl acetate aq, 5% uranyl acetate in methanol, 0.75% uranyl formate, 1% phophotungstic acid, Reynold’s lead citrate, 0.6% formvar, TCH, HMDS and many other EM-related chemicals available on request.
- Epoxy Resins –TAAB TLV low viscosity, Agar 100 and Durcupan.
- Acrylic resins - LR White and Lowicryl HM20
- Recommended reading - Winey, M., Meehl, J.B., O'Toole, E.T. and Giddings, T.H., 2014. Conventional transmission electron microscopy. Molecular biology of the cell, 25(3), pp.319-323.
Cryo-fixation for TEM:
- Leica EM PACT2 and EM ICE High Pressure Freezers (HPF) - Enables cells and tissue to be cryo-fixed using liquid nitrogen under high pressure, which prevents the formation of ice crystals and preserves cells as close as possible to their native state without the artifacts that can arise from chemical fixation or plunge freezing. The EM PACT2 is transportable and freezes samples up to 1.2 mm wide and 200 µm thick. The new, user-friendly EM ICE freezes samples up to 6 mm wide and 400 µm thick, as well as sapphire discs for correlative light and electron microscopy.
- Leica AFS2 Automated Freeze Substitution Unit with FSP robot attachment - The AFS2 performs automated freeze substitution of cryo-fixed samples, which occurs at very low temperatures to minimise ice crystal fromation and extraction of proteins. Resin infiltration can also be performed in the AFS and blocks cured with UV light at -45 °C to preserve GFP fluorescence and/or the antigenicity of samples for subsequent immunogold labelling. We also have the FSP robot attachment available allowing automated ethanol dehydration (for the PLT technique), resin infiltration and polymerisation of samples, which saves a lot of manual processing time.
- FEI Vitrobot Mark IV - For automated vitrification of samples on TEM grids for cryo-TEM.
- Recommended reading - McDonald, K. and Morphew, M.K., 1993. Improved preservation of ultrastructure in difficult‐to‐fix organisms by high pressure freezing and freeze substitution: I. Drosophila melanogaster and Strongylocentrotus purpuratus embryos. Microscopy research and technique, 24(6), pp.465-473.
Microwave processing:
- Leica AMW Automated microwave processing unit – The use microwaves during the EM preparation procedure greatly facilitates the penetration of reagents, thereby significantly shortening the processing time. Conventional TEM processing takes 3-5 days, depending on the sample and protocol used, but with microwave-assisted processing the entire procedure from fixation to resin polymerisation can be completed in half a day. The Leica AMW makes microwave processing of biological samples even easier by automating the process. All you need to do is load the samples into baskets, load the reagent vials and the program and it does everything else for you! Aside from being very fast, microwave processing is also a good option to try with samples that are traditionally difficult to infiltrate, such as cartilage and plant tissue.
- Recommended reading - Wendt, K.D., Jensen, C.A., Tindall, R. and Katz, M.L., 2004. Comparison of conventional and microwave‐assisted processing of mouse retinas for transmission electron microscopy. Journal of Microscopy, 214(1), pp.80-88.
Ultramicrotomy:
- Leica UC7 and UCS - For semithin (500 nm) and ultrathin (70 nm) sectioning of resin-embedded specimens for light microscopy (LM) and TEM, respectively. The UC7 model has a digital touch-screen control panel and brightness-controlled LED lighting for improved knife-block illumination. The top-mounted digital camera and HD-TV allow the sectioning process to be easily demonstrated to users during training sessions.
- Leica KMR3 knifemaker - This produces high quality glass knives for ultramicrotomy. The KMR3 model is compact and easy to use. Glass knives are used for semithin and ultrathin sectioning by users new to ultramicrotomy and, as the user becomes more experienced, can be replaced by a diamond knife.
- Recommended reading - Hagler, H.K., 2007. Ultramicrotomy for biological electron microscopy. Electron Microscopy: Methods and Protocols, pp.67-96.
SEM sample preparation:
- Touismis AutoSamdri 815 Critical Point Dryer – This semi-automated instrument dries ethanol dehydrated samples without the surface tension artifacts that usually occur by air or chemical drying. The AutoSamdri 815 takes samples through several automated flushing steps that replace the liquid medium (usually ethanol) with pressurized liquid CO2, which is then heated to its critical point so that it vapourises without a gas/liquid interface (and therefore without surface tension forces that can damage fine surface structure).
- Quorum Technologies Q150R ES Dual Carbon/Sputter Coater – The Q150R ES is a compact rotary-pumped dual coating system. It can be used as an evaporative carbon coater to coat SEM samples or filmed TEM grids with a thin layer of carbon. There is also a glow-discharge attachment available to ensure carbon-coated grids are ‘wettable’ prior to use with negative staining techniques. It can also be used as a sputter coater, to deposit a fine layer of Au or Au/Pd onto SEM samples to increase conductivity.
- Recommended reading - Heckman, C., Kanagasundaram, S., Cayer, M. and Paige, J., 2007. Preparation of cultured cells for scanning electron microscope. Nature Protocols Network.
Negative staining:
- Leica EM ACE200 Carbon coater & Glow discharge unit - There is also a glow-discharge attachment available to ensure carbon-coated grids are ‘wettable’ prior to use with negative staining techniques.
- Stains – We have ready-made stocks of uranyl acetate, uranyl magnesium acetate and uranyl formate, and other negative stains (phosphotungstic acid, sodium silica tungstate) are available upon request.
- Recommended reading - Ohi, M., Li, Y., Cheng, Y. and Walz, T., 2004. Negative staining and image classification—powerful tools in modern electron microscopy. Biol Proced Online, 6(1), pp.23-34.
Protein localisation:
- Immunolabelling – We have extensive experience with immunogold labelling and our preferred method is post-embedding labelling, ie; on sections of high pressure frozen samples which have been processed into resin at low temperature for better antigenicity preservation. Pre-screening of the primary antibody with immunofluorescence is critical to success, as not all antibodies work well at the EM level or require a higher concentration for specific labelling. We also have a wide range of gold conjugated secondary antibodies available for use. Recommended reading: McDonald, K., 1999. High-pressure freezing for preservation of high resolution fine structure and antigenicity for immunolabeling. Electron Microscopy Methods and Protocols, pp.77-97.
- Genetic tags for EM – An alternative option to immunolabelling (for instance if no antibody is available) is to fuse your protein of interest to a genetic tag specifically developed for EM, of which there are now several, see: Ellisman, M.H., Deerinck, T.J., Shu, X. and Sosinsky, G.E., 2012. Picking faces out of a crowd: genetic labels for identification of proteins in correlated light and electron microscopy imaging. Methods in Cell Biology, 111, p.139.
Correlative light and electron microscopy (CLEM) – This is a powerful technique which allows fluorescent proteins of interest to be placed in ultrastructural context by imaging the same sample with two or more techniques. A nice overview of CLEMis given by: De Boer, P., Hoogenboom, J.P. and Giepmans, B.N., 2015. Correlated light and electron microscopy: ultrastructure lights up!. Nature methods, 12(6), pp.503-513.
We have also developed a method here for super-resolution CLEM. This method works very well in culture cells highly expressing GFP, YFP/mVenus or mRuby2. Please see: Johnson, E., Seiradake, E., Jones, E.Y., Davis, I., Grünewald, K. and Kaufmann, R., 2015. Correlative in-resin super-resolution and electron microscopy using standard fluorescent proteins. Scientific reports, 5.
Sample Prep
Sample Prep